Przegląd
Akumulatory litowe są teraz używane w wielu dziedzinach, i w przeszłości, akumulatory kwasowo-ołowiowe, Akumulatory kadmu, a baterie nikiel były używane w tych polach. Ten artykuł wprowadzi historię rozwoju akumulatorów litowo-jonowych i powiązaną wiedzę o strukturze baterii litowo-jonowej, skierowane do czytelników, którzy są zainteresowani lub mają potrzeby zakupowe dla baterii litowo-jonowych, Aby pomóc im dokonywać mądrzejszych wyborów przy zakupie baterii.

1. Historia rozwoju akumulatorów litowo-jonowych
W ostatniej dekadzie, Akumulatory litowo-jonowe stały się dominującym akumulatorami chemicznymi baterii w prawie wszystkich branżach. W porównaniu z wcześniej popularnymi materiałami chemicznymi (akumulatory kwasowo-ołowiowe, akumulatory niklowo-kadmowe, i baterie alkaliczne), Akumulatory litowo-jonowe są lepsze pod wieloma aspektami. Lit jest obecnie materiałem chemicznym o najwyższej użytej gęstości energii, i z dodatkowymi funkcjami, Może stać się najbezpieczniejszym materiałem chemicznym. Litowa energia jest aktywną dziedziną badawczą, Dlatego każdego roku opracowywane są nowe materiały chemiczne.
Pojęcie akumulatorów litowo-jonowych zostało po raz pierwszy zaproponowane w latach siedemdziesiątych, Kiedy brytyjski chemik Stanley Whittingham wynalazł baterię, która z czasem mogłaby się ładować. Próbował użyć tytanu disiarczkowego i litu metalu jako elektrod, Ale to spowodowało, że bateria zwarła i eksplodowała.
Kwestie bezpieczeństwa akumulatorów litowo-metalowych spowodowały rozwój akumulatorów litowo-jonowych. Chociaż akumulatory litowe mają większą gęstość energii, Akumulatory litowo-jonowe są bardzo bezpieczne podczas ładowania i rozładowywania przy użyciu określonych wytycznych bezpieczeństwa.
W latach 80, John Goodenough i Akira Yoshino dalej eksperymentowali, aby akumulatory były bezpieczniejsze. Rozpoczął się rozwój akumulatorów litowo-jonowych.
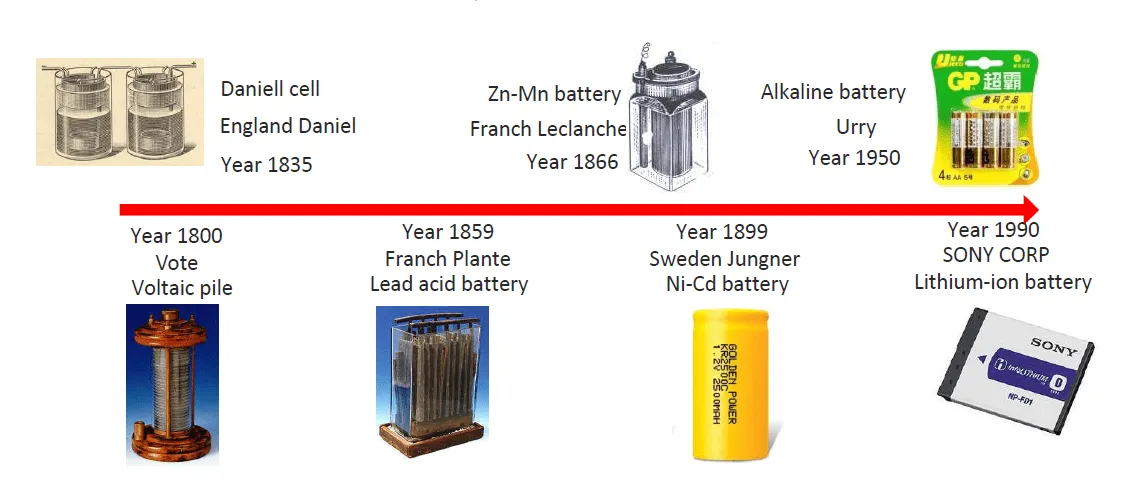
W latach 90, Technologia litowo-jonowa zaczęła być faworyzowana i szybko spopularyzowana. Ówcześnie, Sony wyprodukował pierwszą partię akumulatorów komercyjnych, Oznaczanie początku komercjalizacji akumulatorów litowo-jonowych. W tym samym czasie, Przenośny rynek urządzeń elektronicznych szybko się rozwija, Wymaganie lekkiej akumulatorów, aby ją zasilać. Baterie litowo-jonowe, jako bezpieczna i mocna bateria, stały się najlepszym wyborem.
W ostatniej dekadzie, Akumulatory litowo-jonowe stały się dominującym akumulatorami chemicznymi baterii w prawie wszystkich branżach. W porównaniu z wcześniej popularnymi materiałami chemicznymi (akumulatory kwasowo-ołowiowe, akumulatory niklowo-kadmowe, i baterie alkaliczne), Akumulatory litowo-jonowe są lepsze pod wieloma aspektami. Lit jest obecnie materiałem chemicznym o najwyższej użytej gęstości energii, i z dodatkowymi funkcjami, Może stać się najbezpieczniejszym materiałem chemicznym. Litowa energia jest aktywną dziedziną badawczą, Dlatego każdego roku opracowywane są nowe materiały chemiczne.
Obecnie, the Pięć najlepszych globalnych firm z baterii litowo -jonowych Czy:
Catl (Chiny)
LG Chem (Korea Południowa)
BYD (Chiny)
Panasonic (Japonia)
Samsung SDI (Korea Południowa)
2. Struktura baterii litowo -jonowej
2.1 Co to jest akumulator litowo-jonowy
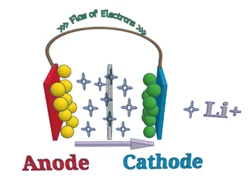
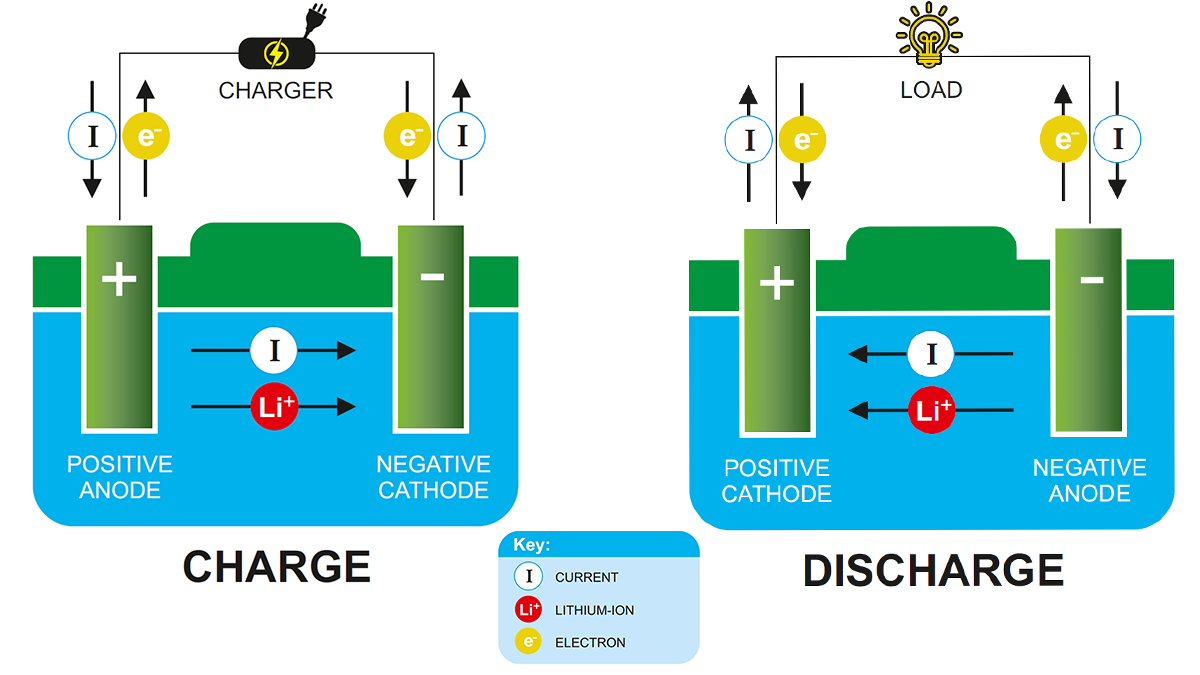
Mówiąc najprościej, A akumulator litowo-jonowy odnosi się do baterii z elektrodą ujemną (anoda) i elektroda dodatnia (katoda), gdzie jony litu są transportowane między dwoma materiałami. Zasada pracy akumulatorów litowo-jonowych jest taka sama jak każda inna akumulator.
Podczas wypisu, Jony litowe przenoszą się z anody do katody i depozytu (osadzać) do elektrody dodatniej złożonej z litu i innych metali. Podczas ładowania, Ten proces jest odwrotny.
Każda akumulator litowo-jonowy ma zakres napięcia, który może działać bezpiecznie. Zakres zależy od składu chemicznego elektrolitu stosowanego w akumulatorze. Na przykład, Akumulatory LFP wynoszą 2,5 V przy 0% stan naładowania (SOC) i 3,6 V przy 100% SOC. Zazwyczaj jest to uważane za bezpieczny zakres działalności akumulatorów LFP, podczas gdy poniżej określonego zakresu jest uważane za nadmierne rozładowanie, i przekroczenie określonych 100% SOC uważa się za nadmierne naładowanie.
2.2 Struktura baterii litowo -jonowej
2.2.1 Anoda
Anoda to elektroda ujemna w baterii. W akumulatorach litowo-jonowych, Anoda zwykle składa się z litu i węgla (zwykle grafitowy proszek). Czystość, Rozmiar cząstek, a jednolity rozkład materiałów anodowych może wpłynąć na ich pojemność i wskaźnik starzenia się.
2.2.2 Katoda
Katoda jest elektrodą dodatnią. W tym miejscu w grę wchodzą różne chemikalia. Katoda określa ogólne właściwości chemiczne energii litowej. Jak anoda, Kolekcjoner jest łączony z materiałem w celu ułatwienia aktywności reakcji elektronicznej. Główna różnica między nimi polega na temperaturze, w której różne chemikalia reagują z elektrolitami (ucieczka termiczna) i wielkość wytwarzanego napięcia.
2.2.3 Elektrolity
Elektrolity umożliwiają przenoszenie jonów litowych i przemieszczanie się między dwiema płytkami. Zazwyczaj, składa się z różnych węglanów organicznych, takie jak węglan etylenowy i węglan dietylu. Różne mieszanki i wskaźniki zależą od środowiska aplikacji baterii.
Na przykład, Do zastosowań w niskiej temperaturze, Lepkość roztworu elektrolitowego będzie niższa niż w przypadku roztworu elektrolitu w temperaturze pokojowej. W bateriach litowych, Litu heksakfosforan (LIPF6) jest najczęstszą solą litową. Można powiedzieć, że najczęściej stosowanym elektrolitem w akumulatorach litowo-jonowych jest litowy heksakosfosforan (LIPF6), którego jakość określa wydajność ładowania i rozładowywania, Life Service, i bezpieczeństwo akumulatorów litowo-jonowych.
Ponieważ LIPF6 ma najlepszą ogólną kompleksową wydajność, Ma doskonałą przyjazność dla środowiska, pasywacja dodatnim kolektora prądu elektrody, aby zapobiec korozji elektrody, i po zmieszaniu z wodą, wytwarza kwas hydrofluorowy (Hf), co sprzyja tworzeniu się folii SEI na elektrodzie ujemnej.
SEI to reakcja chemiczna między metalem litowym a elektrolitem, który tworzy stałą warstwę elektrolitu na powierzchni litowego metalu. Odgrywa rolę w izolacji i ochronie między metalem litowym a elektrolitem.
W normalnych warunkach, Producenci akumulatorów zazwyczaj ładują się powoli, tworząc jednolity SEI na anodzie węglowej.
2.2.4 Membrana
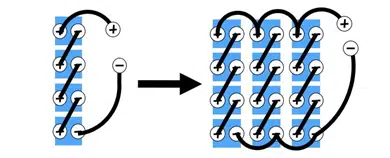
Separator akumulatorów litowo-jonowych jest porowatą folią plastikową, która ułatwia zapobieganie bezpośredniemu kontaktowi między anodą a katodą. Te cienkie folie są zwykle 20 Mikony grube z małymi porami, które umożliwiają przechodzenie jonów litowych podczas procesów ładowania i rozładowywania. Gdy bateria przekroczy zakres temperatur lub doświadczy zwarcia, Ten separator zamyknie pory i zapobiegnie przejściu jonów litowych, w ten sposób zatrzymując reakcję chemiczną.
3. Zalety baterii litowo-jonowych
3.1 Zalety struktury baterii litowo -jonowej
1. Wyładowanie wysokiej prędkości, stabilna pojemność
2.Szybkie ładowanie
Baterie litowo -jonowe - naładowane w środku 1 godzina
Baterie ołowiowe - nad 9 godziny
3.Mały ślad i silna pojemność obciążenia
4.Wiele cykli i długie życie
Baterie litowo -jonowe - żywotność cyklu jest zwykle 5000 czasy, a całkowite rozładowanie nie wpływa na żywotność cyklu
Akumulatory kwasowo-ołowiowe -300 Do 500 czasy, Całkowite zwolnienie wpłynie na ich żywotność
5.Wysoka wydajność energetyczna
Baterie litowo-jonowe -96% wyjście, 4% Utrata ciepła
Akumulatory kwasowo-ołowiowe -15% Utrata ciepła w 85% wyjście
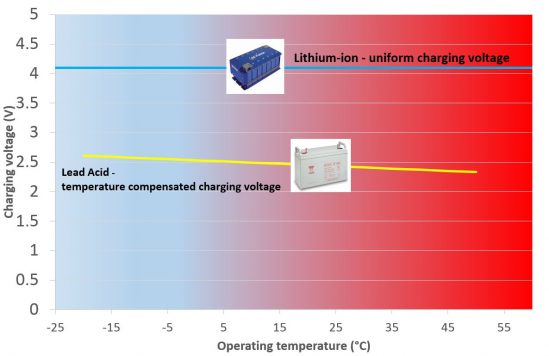
6.Szeroki zakres napięcia ładowania
Nie wymaga kompensacji napięcia
7.Zmniejsz koszty zarządzania termicznego
Baterie litowo -jonowe - akceptowalny cyrkulacja powietrza
Bateria ołowiu - wymaga klimatyzacji
8.Brak emisji gazu
Akumulatory litowo -jonowe - działanie w uszczelnionych pojemnikach
Baterie kwasowe ołowiu - wymagają wentylacji wodorowej
9.Nie toksyczne, Brak ograniczeń recyklingu
Zielona nowa energia, bezpieczne w użyciu.
Aby uzyskać więcej porównania między baterią litową a baterią kwasową ołowiową, Kliknij, aby wyświetlić: Bateria litowa vs.. Akumulator kwasowo-ołowiowy Aby uzyskać bardziej szczegółowe i konkretne treści.
3.2 Powód, dla którego wybierają wymianę akumulatorów kwasowych ołowiu na akumulatory litowo-jonowe
3.2.1 Poprawa wydajności
Dzięki postępom w BMS i technologii ładowania, Sprzęt zasilający akumulator litowo-jonowy może pomóc poprawić wydajność i skrócić przestoje spowodowane potrzebą ładowania sprzętu zasilanego baterią.
3.2.2 Poprawa wydajności
Operatorzy nie muszą się martwić o problemy z ładowaniem urządzeń, a technologia akumulatorów litowo-jonowych pozwala firmom inwestować w rozwiązania automatyki, Zmniejszenie kosztów dla firm.
3.2.3 Prostszy sposób ładowania i przechowywania
Akumulatory litowo -jonowe mogą być naładowane w dowolnym momencie, co oznacza, że możesz je obciążyć ich wygodą. Akumulatory litowo -jonowe również nie wymagają własnej przestrzeni ładowania lub przechowywania, ponieważ nie stanowią ryzyka środowiska, takich jak akumulatory ołowiowe.
3.2.4 Brak wymagania konserwacji
W przeciwieństwie do akumulatorów ołowiowych, Akumulatory litowo-jonowe nie wymagają żmudnych kontroli i metod konserwacji.
3.2.5 Poprawa bezpieczeństwa operacyjnego
Akumulatory litowo -jonowe poprawiają bezpieczeństwo operacyjne obiektów za pomocą różnych środków, są również bardziej przyjazne dla środowiska ze względu na niższe ryzyko przegrzania, eksplozja, lub emisja szkodliwych gazów lub cieczy.
4.1 Zrównoważone ładowanie
Nałóż akumulator po pełnym cyklu ładowania powyżej normalnego napięcia. Ten krok jest niezbędny, aby pomóc usunąć nagromadzone siarczany i zrównoważyć napięcie każdej baterii w akumulatorach ołowiowych.
4.2 Degradacja baterii
Proces zmniejszania ilości energii, którą bateria może przechowywać. Temperatura, ładowanie i rozładowywanie napięcia, a ładowanie i głębokość rozładowywania może wpływać na stopień, w jakim z czasem maleje pojemność baterii.
4.3 Liczba cyklu akumulatora
Jeśli akumulator uzupełnia jedno ładowanie i rozładowanie jako cykl, skumulowana liczba ładunków i zrzutów. Cykl akumulatora składa się z 100% rozładowanie i ładowanie.
4.4 Żywotność baterii
Jak długo można użyć baterii w ramach jej żywotności. Życie jest mierzone przez liczbę pełnych cykli ładowania i rozładowywania.
4.5 Temperatura pracy
Akceptowalna temperatura otaczającego środowiska, w którym działa bateria. Jeśli temperatura robocza przekracza zakres, Bateria może się nie powieść.
4.6 UL Listing/Certification
Lista/certyfikacja UL oznacza, że UL ocenił próbki produktów, aby upewnić się, że spełniają określone wymagania. Obejmuje to testowanie próbek obejmujących funkcjonalne bezpieczeństwo i przypadki użycia struktury baterii litowo -jonowej.
5. Dlaczego warto wybrać jony litowe- Analiza z perspektywy struktury baterii litowo -jonowej

5.1 Doskonała jakość
Najważniejsze są wysokie gęstość energii i rozległe cykle rozładowania akumulatorów litowo-jonowych, czyniąc je niezbędnymi w wielu urządzeniach. I są również lepsze od tradycyjnej chemii baterii w wielu innych aspektach. Oprócz wysokiej gęstości energii baterii, Mogą również rozładować przy dużej mocy i szybko ładować. Daje im to większą elastyczność operacyjną niż akumulatory ołowiowe.
W scenariuszach aplikacji, w których moc lub czas ładowania jest ciasny, takie jak systemy fotowoltaiczne słoneczne, Ciągłe działanie w częściowo naładowanych stanach nie spowoduje szkody dla akumulatorów litowo-jonowych.
5.2 Przyjazny dla środowiska
Interakcja między akumulatorami litowo-jonowymi a środowiskiem jest bardzo łagodna. Podczas ładowania nie są emitowane szkodliwe gazy, a utrata ciepła jest bardzo niska. Oznacza to, że akumulatory litowo-jonowe mogą być stosowane w zamkniętych przestrzeniach, całkowicie odizolowane od otoczenia. Recykling i ponowne wykorzystanie odrzuconych baterii jest również bardzo wygodne, ponieważ nie zawierają toksycznych substancji, takich jak kadm, rtęć, i ołów.
5.3 Wiele rodzajów struktur
Podczas wypisu, Ładunek przesuwa się przez obwód zewnętrzny między elektrodami akumulatora. W celu zrównoważenia transferu ładowania w baterii, Pozytywnie naładowane jony litowe przesuwają się przez wewnętrzny obwód elektrolitowy między elektrodami dodatnimi i ujemnymi. Podczas ładowania, proces jest odwrotny, a jony litowe powracają przez elektrolit.
Jako katody można stosować wiele rodzajów chemikaliów (katody) do produkcji materiałów elektrodowych przenoszących jony litowe. Materiały elektrolitowe są również kierunkiem badawczym, a stan materiałów, takich jak ciśnienie stałe i cieczy, jest również tematem badawczym. To bardzo aktywna dziedzina badań i rozwoju, który napędza rozwój akumulatorów litowo-jonowych w coraz większej liczbie aplikacji rynkowych.
Wniosek
Teraz, gdy zrozumiałeś tyle analiz i danych, Powinieneś mieć pewne zrozumienie baterii litowych: ich historia, zalety, i kierunek rozwoju.
Nadeszła przyszłość produktów elektrycznych. Zapotrzebowania na przejście z tradycyjnej energii na nową energię nie można zignorować. W miarę jak coraz więcej branż i przedsiębiorstw zdaje sobie sprawę z zalet technologii akumulatorów litowo-jonowych, Podejmowanie decyzji o zmianie koncentracji biznesowej stało się łatwiejsze.
Skontaktuj się z nami, Gycx One-Stop Service zapewnia najdoskonalsze rozwiązanie dla twoich potrzeb.
Czy planujesz teraz zainwestować w technologię akumulatorów litowo-jonowych?
Często zadawane pytania
1. Czy akumulatory litowe mogą wymieniać baterie alkaliczne?
Chociaż baterie litowe wykorzystują droższą technologię akumulatorów, Ich zdolność do utrzymania wysokiego napięcia oznacza, że są doskonałą alternatywą dla akumulatorów alkalicznych.
2.Czy akumulatory litowe wyciekają?
Akumulatory litowe nie wyciekają, więc są bardzo bezpieczne do przechowywania.
Lit może zapalić się w kontakcie z powietrzem lub wodą. Mniej prawdopodobne są wycieki, ponieważ w przypadku ciekłych elektrolitów, Technologia leczenia wydechowego jest już bardzo dojrzała.
3.W jakiej temperaturze eksplodują akumulatory litowo-jonowe?
Baterie litowe mogą eksplodować o 538 stopni Celsjusza.
Jeśli bateria litowa jest podgrzewana przez długi czas, to może. Ponieważ akumulatory litowo-jonowe mają bardzo wysoką energię, Kiedy stają się gorące, uwalniają rozpuszczalniki organiczne, które działają na elektrolit; To ciepło może powodować wybuch.
Zwarcia, które występują, gdy zaciski akumulatorowe zetkną się z metalem, mogą również powodować wybuchy.
