Overview
Solid state lithium ion batteries is an emerging battery technology that has several significant advantages over traditional liquid lithium-ion batteries. This also makes solid-state lithium-ion batteries regarded as the next generation of battery technology. Although Solid State Lithium ion Batteries still have many areas for improvement in terms of safety, power, and energy density compared to the most advanced lithium-ion batteries available today, such as relying on the discovery and application of high-quality solid electrolytes to replace the liquid solutions currently used.
The lithium battery industry is constantly developing, conducting research every day to develop increasingly high-performance innovative technologies to ensure that this product has more range, greater power, and shorter charging time.
In this sense, solid-state battery technology seems to be the last frontier of technology, and this emerging solution has great potential to become the future of electric vehicles. It has a series of huge advantages, but also many limitations that are delaying its entry into the market.
What is a Solid state lithium ion batteries?
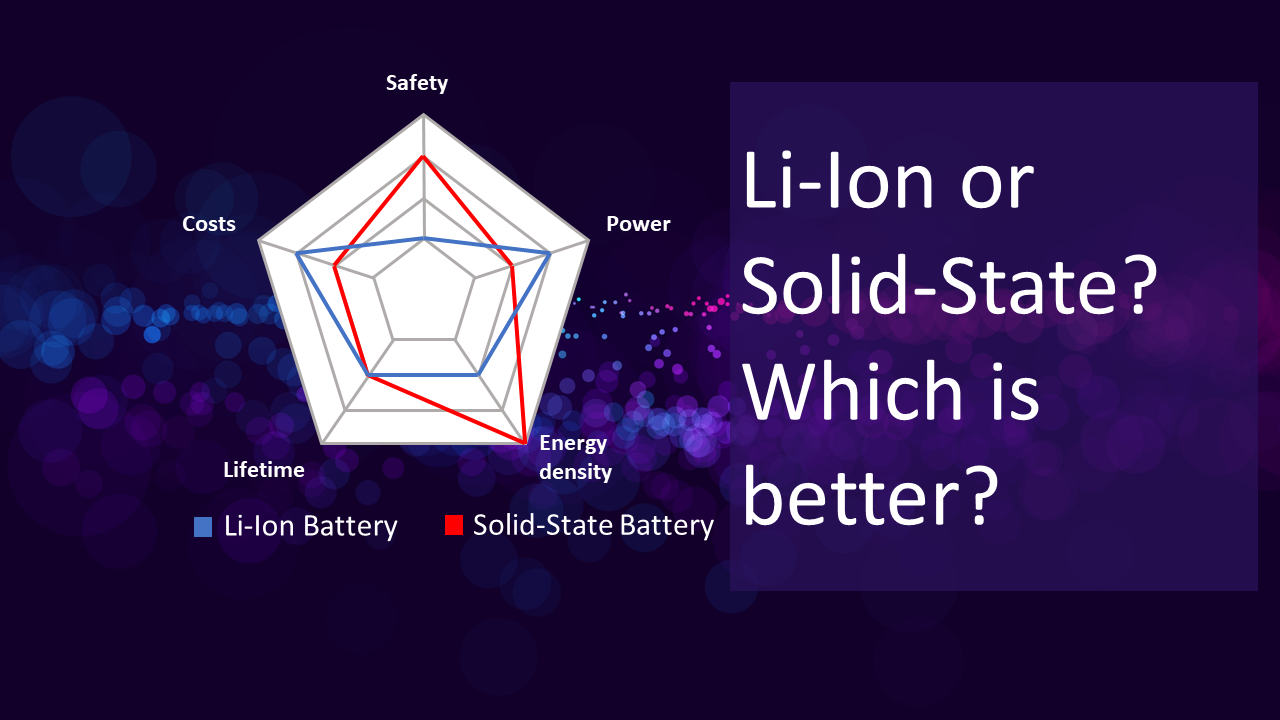
Direct answer: Both are lithium batteries, but it would be better.
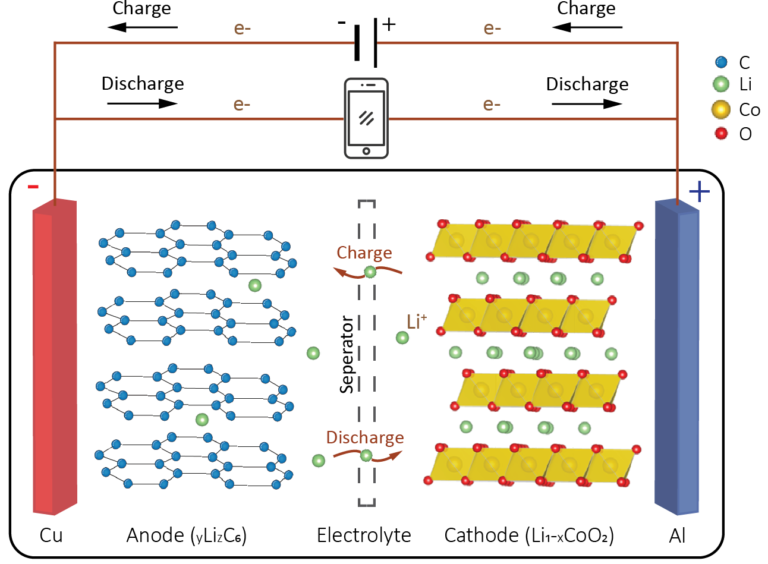
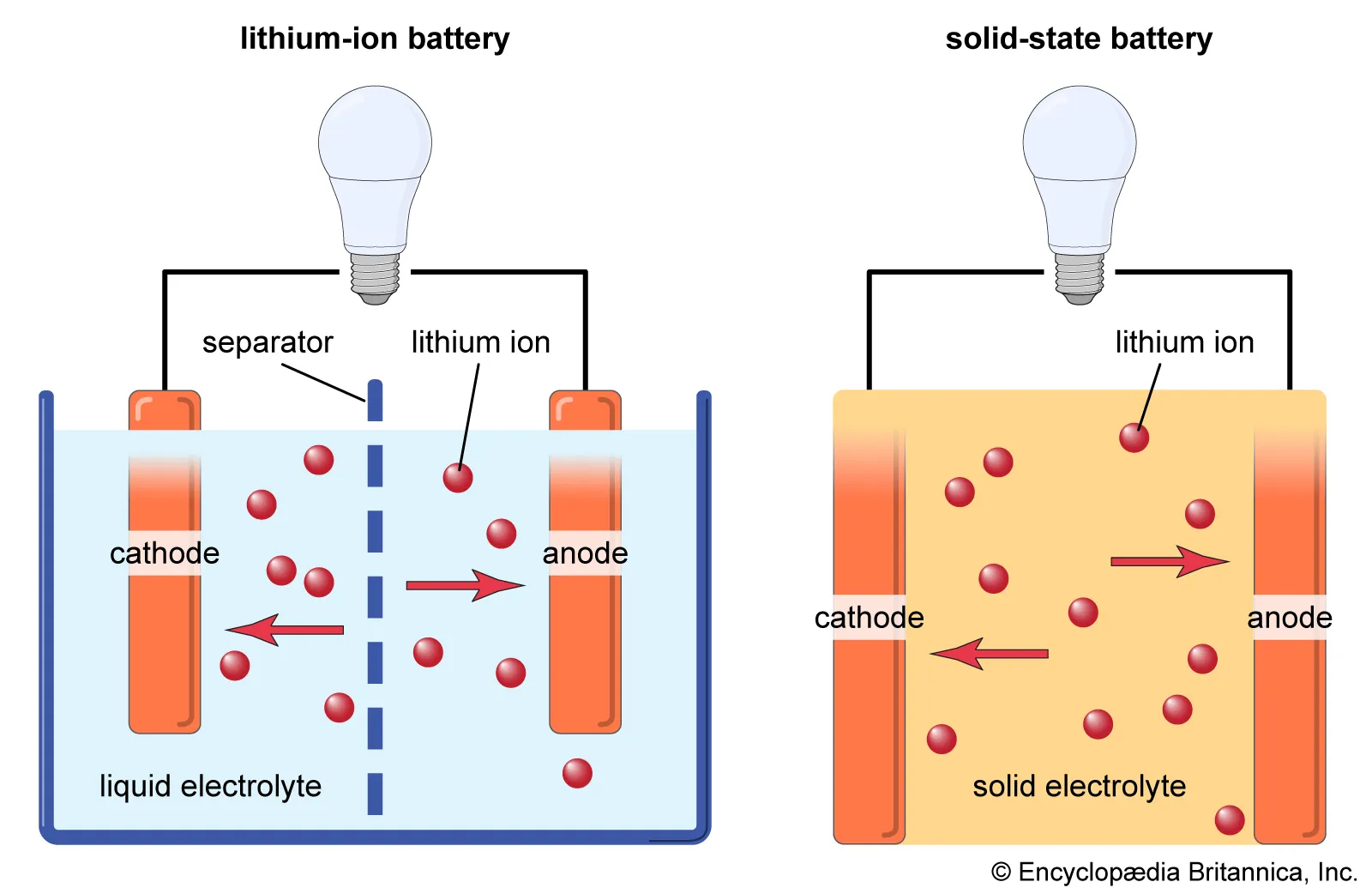
Their working principles are the same, except that there is no liquid component inside the solid-state lithium battery. In traditional lithium-ion batteries, the electrolyte is liquid. It is generally composed of lithium salts (such as LiPF 6, LiBF 4, or LiClO 4) dissolved in organic solvents (such as ethylene carbonate, dimethyl carbonate, etc.). There is a liquid component called electrolyte in the battery of a smartphone, which allows lithium ions to flow freely and provide power to your device after charging. This also leads to a phenomenon called liquid leakage when you drop your phone.
Solid State Battery vs Lithium Ion: Revealing Differences
In the development of battery energy storage, two major competitors are vying for the top position: solid-state batteries and lithium-ion batteries. These powerful batteries power our Consumer Electronics and renewable energy systems. With the emergence of solid-state battery technology, the battery industry is undergoing a technological revolution, challenging the dominant position of lithium-ion batteries.
But what is the key difference between solid-state batteries and lithium-ion batteries?
As mentioned before, the main difference lies in their electrolyte composition, which can also be seen from their names. Besides, we can learn more. It can also be understood as, why study solid-state lithium batteries? What are its benefits?
Energy density
Solid state lithium ion batteries: This type of battery can hold almost twice as much energy as liquid lithium-ion batteries, especially when replacing anode materials with smaller ones.
Lithium ion battery:The capacity of lithium-ion batteries composed of different chemical raw materials may vary, but compared to solid-state batteries, their energy density is lower.
Electrolyte material
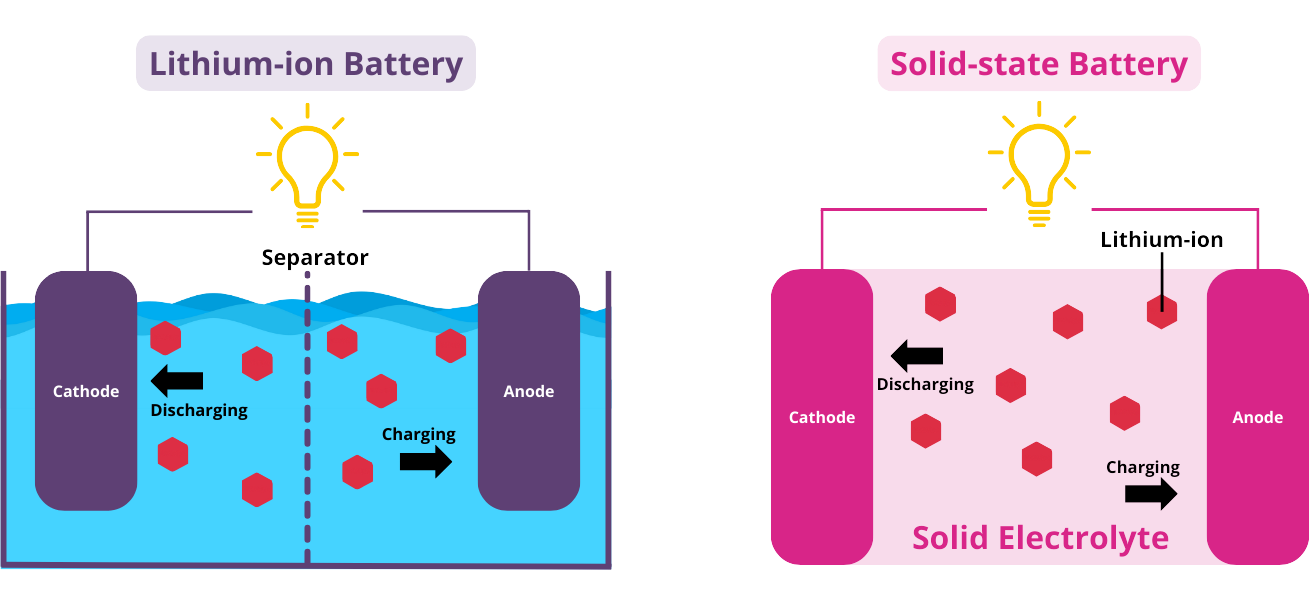
The electrolyte of a battery is a conductive chemical mixture that allows metal ions to flow between the anode and cathode, resulting in electrochemical reactions.
The main difference between common lithium-ion batteries and solid-state lithium batteries on the market is that the former uses a liquid electrolyte to regulate the current, while solid-state batteries choose a solid electrolyte.
Solid state lithium ion batteries: Using solid electrolytes instead of liquids results in a lighter overall weight and higher energy density. This will also have some weight advantages in some modes of transportation.
Lithium ion battery: Electrochemical reactions occur in liquid electrolytes, causing lithium ions to flow between the cathode and anode.
Lithium ion batteries have different chemical types, and there are also differences between them. There are LCO, LMO, LFP, NCA, LTO, and so on. You can read this article titled “6 Chemical Types of Lithium ion Batteries that You Can Choose From” to get a more detailed understanding.
Lithium battery safety
Solid state lithium batteries: Solid electrolytes reduce the risk of thermal accidents, making them safer, not only in transportation but also in use.
Lithium ion battery: Easy to encounter safety issues such as overheating, expansion, and fire, posing a greater risk than lead-acid batteries.
How Long Does Lithium Battery Last
Solid state lithium batteries: Solid electrolytes have higher stability and lower reactivity compared to liquids, resulting in a relatively longer service life.
Lithium ion battery: The number of times a battery can be charged is limited within a certain range. Liquid lithium-ion batteries have slightly higher temperature requirements, and the ambient temperature for charging, discharging, and use is usually indicated. It has a shorter lifespan compared to solid-state batteries.
Structure of solid-state batteries
Every lithium-ion battery has:
Two electrodes, which are compounds capable of accepting lithium ions embedded in their structure.
Cathode refers to the positive electrode of a battery made of cathode materials such as LFP, NMC, LMO, etc.
Anode refers to the negative electrode of a battery made of an anode material (such as non active substances like carbon or graphite).
The central partition, which is a thin layer made of plastic polymer (polyethylene or polypropylene), serves as a partition between the anode and cathode, as well as an insulator.
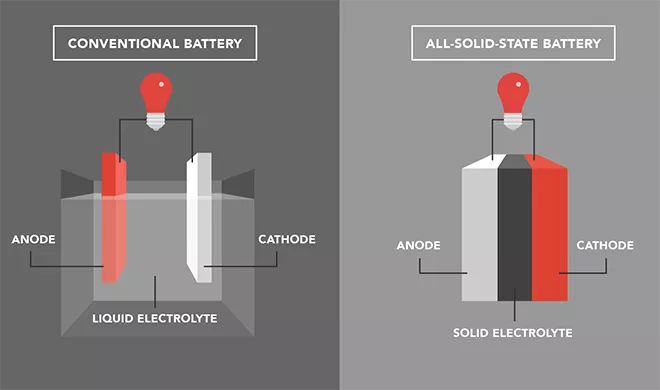
Electrolyte: a medium for ion movement; Organic liquids containing lithium salts. Electrolyte fills the entire volume inside the battery, immerses the electrodes, and allows lithium ions to move.
In existing lithium-ion batteries, the main function of the separator is insulation, but it has no other functions. It is completely saturated with liquid electrolyte. The anode is usually made of graphite, and lithium ions move through the electrolyte and are inserted into the crystal structures of the anode and cathode. These structures have gaps inside that can accommodate extremely small lithium ion particles.
However, the internal structure of solid-state batteries is different because all of its components and media are solid.
Solid state batteries consist of the following components:
Anode: Made of lithium metal (pure lithium).
Cathode: Made of the same compound as lithium-ion batteries (such as LFP, NMC, LMO, etc.).
Diaphragm, usually ceramic or solid polymer, is also used as an electrolyte.
The gray layer in the middle is a solid separator, which not only serves as a separator between the anode and cathode, but also as an electrolyte.
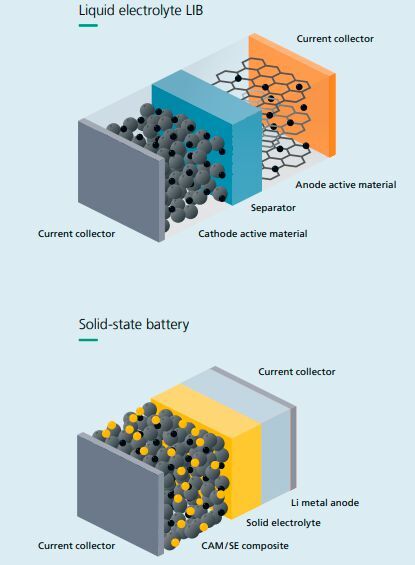
Therefore, it is both a medium for ion movement and has electrical insulation function, serving as a mechanical membrane between the anode and cathode. This sturdy and durable support can remove the graphite structure of the anode, ensuring that lithium metal accumulates directly on the anode. In addition, there are some semi-solid solutions, in which the electrolyte is a gel like substance.
It can be seen that the concept of solid-state batteries is superior in terms of the space and material application of electrolytes and separators.
How do Solid state lithium ion batteries work?
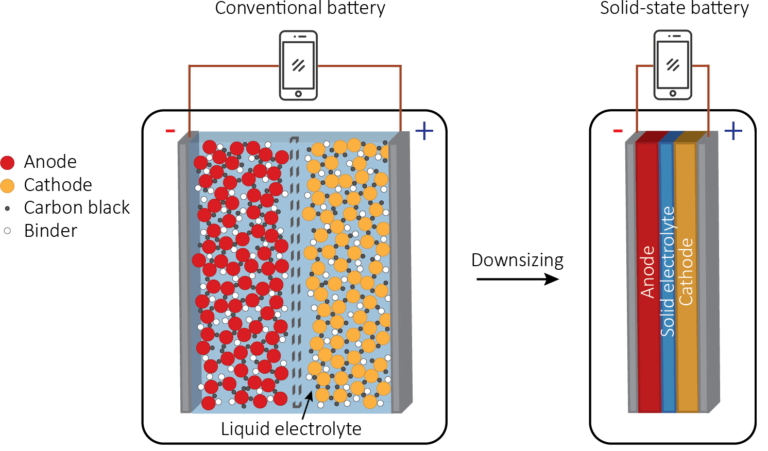
The working principle of solid-state batteries is very similar to traditional lithium-ion batteries, except that they use solid electrolytes instead of liquid electrolytes through which lithium ions flow. The biggest advantage of doing this is that solid-state batteries do not have all the safety hazards of liquid electrolytes.
But the basic principle of operation is the same. In solid-state batteries, pure lithium accumulates at the positive electrode of the battery, and then flows from the negative electrode to the positive electrode during discharge, accumulating in the form of metal instead of containing metal oxide electrodes like standard liquid electrolyte batteries. This feature not only makes the battery safer, but also saves a lot of space.
What are the advantages of solid-state batteries?
Product size
Solid state electrolytes have replaced the separators in traditional lithium-ion batteries, taking up less space and making them lighter than regular lithium-ion batteries. The breakthrough in technology has the potential to be applied to fields such as airplanes and trucks for transportation.
Battery weight
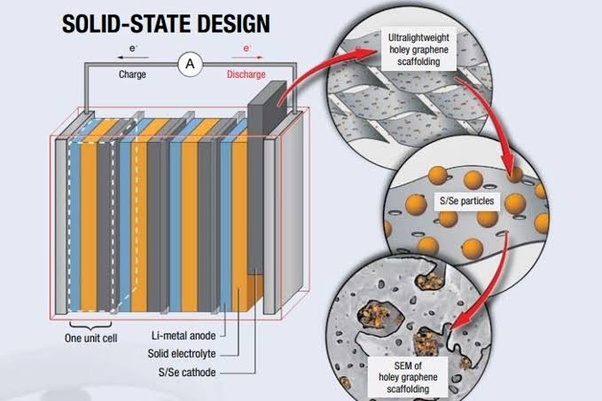
Lithium is the lightest metal element, which allows lithium metal anodes in solid-state batteries to provide higher energy density in smaller packages. In this way, solid-state batteries have become a lightweight option.
For example, as electric vehicles continue to grow in size, the required battery capacity is also increasing in order to maintain range data, which also brings about the problem of increased weight. Because the increased weight will lead to increased tire wear, resulting in more particulate pollutants. Therefore, reducing the weight of electric vehicles and their batteries not only helps to reduce exhaust emissions, but also reduces tire wear and particle release. Solid state batteries can provide excellent basic requirements for it.
Safety and duration of use

Lithium ion batteries contain volatile and flammable liquid electrolytes, which pose a risk of fire. Solid state batteries, on the other hand, can withstand higher temperatures and have stronger thermal stability, making them safer.
Due to its smaller size and higher energy density, solid-state batteries can store more energy in a smaller space, which means that using them can improve battery life.
A manufacturer claims that their electric vehicle can travel 745 miles on a single charge.
In terms of charging speed, solid-state batteries are also excellent. Lithium ion batteries in electric vehicles typically take 20 minutes to 12 hours to fully charge, while solid-state batteries can be charged to at least 80% of their capacity in just 10 to 15 minutes.
Solid state batteries also have a longer lifespan and can be charged up to 5 times more than lithium-ion batteries, thereby extending the overall lifespan of the battery. Data comparison shows that Solid state lithium batteries are superior.
Reduce carbon footprint
Solid state batteries use fewer materials and can reduce climate impact by 39% compared to lithium-ion batteries. This means it is also more environmentally friendly and in line with the development concept of carbon neutrality.
Fast charging
The latest research has found that the charging speed of solid-state batteries is six times faster than existing lithium-ion charging technologies. But in order to achieve this speed, some other key performance indicators may be sacrificed, so further optimization is needed.
However, it can be confirmed that liquid electrolytes are prone to damage at high temperatures, while solid electrolytes perform better at high temperatures. This means that solid-state batteries can better perform during fast charging and heat generation, and can also be considered to not lose their own performance in terms of heat generation.

Why do we need them?
Through the introduction of Solid State Lithium ion batteries, analysis of the differences between Solid State Battery and Lithium Ion Battery, and the advantages of Solid State Battery, we have gained a comprehensive understanding.
Why do we need them?
Traditional liquid electrolyte lithium batteries must have a considerable volume to power large equipment such as cars. And these batteries have safety hazards, they may expand due to temperature changes or leak when subjected to excessive compression. It should be noted that the liquid inside is flammable.
Everyone has experienced the anxiety of “mobile phone batteries running low” and understands that the issue of battery life during use is also a factor.
Although traditional lithium-ion batteries have improved compared to previous batteries, they still have shortcomings in addressing these issues. Slow charging speed and limited lifespan make them perform poorly in many applications.
And solid-state batteries are gradually solving these problems. They have a smaller volume but larger capacity, lighter weight, and higher safety. The charging speed is faster and the lifespan is longer, so it can greatly compensate for the shortcomings of traditional lithium batteries. That’s also why we need them.
When will we be able to see solid state lithium ion batteries
Solid state technology has been used in small quantities in the following fields:
Batteries suitable for working in suitable climates
Aerospace application battery
Semi solid or solid hybrid battery.
A Chinese car company recently launched 50 cars equipped with semi-solid state batteries
But Solid state batteries are still under development, and there are still some challenges to overcome in order to be commercially applied on a large scale.

Cost
At present, the production cost of solid-state batteries is higher than that of ordinary lithium-ion batteries because they use more expensive materials and the production process is more complex. Usually, mature market technologies are optimized before they are put into use, so this is still an ongoing process.
Scale up
Most of the development of solid-state batteries is still in the laboratory stage, and solid-state batteries are considered safer than traditional batteries. However, the problem of short circuit risk caused by lithium metal needle like growth still needs to be studied and solved urgently. Meanwhile, how to expand production scale is also an ongoing research topic.
Stability issues
Solid state batteries are like breathing during the charging and discharging process. Lithium metal anodes become thicker during charging and thinner during discharging. The main issue lies in how to maintain both its fixed and compressed state simultaneously.
The battery must remain compressed to ensure that the internal layers do not separate, but simply fixing it to the outer shell is not enough, as the battery requires flexible stretchability when “breathing”.
Therefore, it is necessary to design a complex mechanical structure. The use of springs to maintain the flexibility of all components during compression, but this mechanical system is complex and expensive, making it difficult to mass produce.
Due to the composition of solid-state batteries, expansion cannot be completely avoided. By conducting research to reduce the demand for pressure, batteries can maintain stability at lower pressures or use more advanced materials to meet the demand. This will be a key direction for future technological development.
Separators and Temperature
Ions are substances that are actually charged atoms, making them easier to move in liquids. To allow ions to move freely in solids, separators (such as ceramic separators) must have special components. At present, we have some high-performance solid electrolytes, but these electrolytes do not perform well at room temperature. They can only become good conductors at temperatures above 50 degrees Celsius.
This imposes limitations on the practical application of solid-state batteries, as batteries in vehicles cannot maintain high temperatures indefinitely.
When the temperature of solid-state batteries is not high, their performance will significantly decrease. Therefore, further research is needed to ensure that solid electrolytes can also perform well at low temperatures, so as to use solid-state batteries in more practical applications.
Research and development in the field of Solid State Batteries are rapidly advancing, and many experts believe that solid-state batteries will eventually become the standard in areas such as electric vehicles.
Conclusion
Many battery industry manufacturers are interested in this promising technology, such as Mercedes Benz, Volkswagen, Toyota, Tesla, etc., and they are investing significant resources in research and development. If technical problems are solved, they will become the first people in the market and thus hold the discourse power. And it is expected to be launched between 2024 and 2026, which is highly anticipated and worth paying attention to.
