Yleiskatsaus
Litiumakkuja käytetään nykyään monilla aloilla, ja menneisyydessä, lyijyakut, kadmium akut, ja nikkeliparistoja käytettiin näillä aloilla. Tämä artikkeli esittelee litiumioniakkujen kehityshistorian ja siihen liittyvän tiedon litiumioniakkujen rakenteesta, suunnattu lukijoille, jotka ovat kiinnostuneita litiumioniakuista tai joilla on tarvetta ostaa niitä, auttaa heitä tekemään viisaampia valintoja akkuja ostaessaan.

1. Litiumioniakkujen kehityshistoria
Kuluneen vuosikymmenen aikana, litiumioniakuista on tullut hallitseva ladattavien akkujen kemiallinen materiaali lähes kaikilla teollisuudenaloilla. Verrattuna aiemmin suosittuihin kemiallisiin materiaaleihin (lyijyakut, nikkeli-kadmium akut, ja alkaliparistot), litiumioniakut ovat monin tavoin parempia. Litium on tällä hetkellä eniten käytetty kemiallinen materiaali, ja muutamilla lisätoiminnoilla, siitä voi tulla turvallisin kemiallinen materiaali. Litiumenergia on aktiivinen tutkimusala, Siksi uusia kemiallisia materiaaleja kehitetään joka vuosi.
Litiumioniakkujen käsite esiteltiin ensimmäisen kerran 1970-luvulla, kun brittiläinen kemisti Stanley Whittingham keksi akun, joka pystyi lataamaan itsensä ajan myötä. Hän yritti käyttää titaanidisulfidia ja litiummetallia elektrodeina, mutta tämä aiheutti akun oikosulun ja räjähdyksen.
Litiummetalliakkujen turvallisuusongelmat ovat saaneet aikaan litiumioniakkujen kehittämisen. Vaikka litiummetalliakuilla on korkeampi energiatiheys, litiumioniakut ovat erittäin turvallisia, kun niitä ladataan ja puretaan erityisten turvallisuusohjeiden mukaisesti.
1980-luvulla, John Goodenough ja Akira Yoshino kokeilivat edelleen tehdäkseen akuista turvallisempia. Litiumioniakkujen kehitys on siis alkanut.
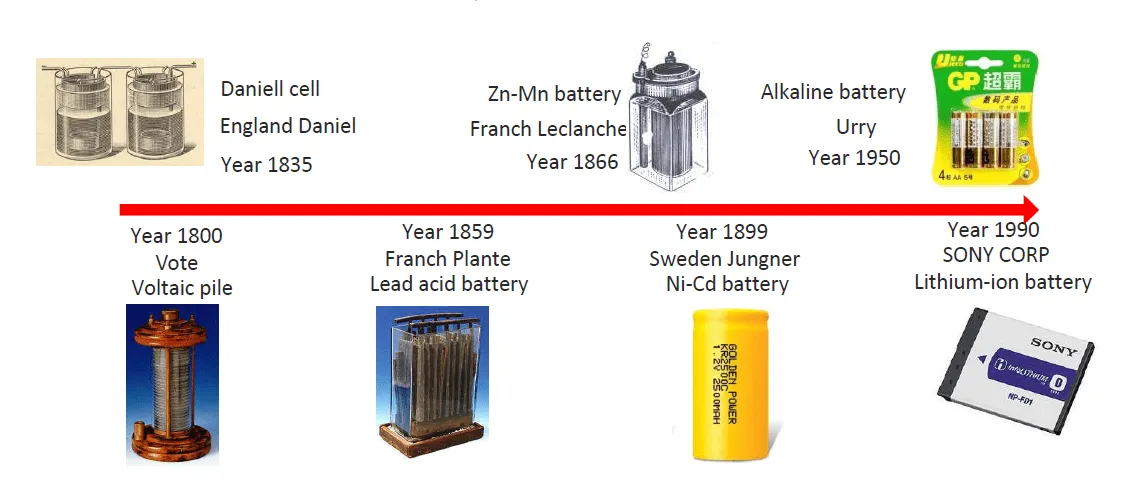
1990-luvulla, litium-ionitekniikkaa alettiin suosia ja suosittu. Tuolloin, Sony valmisti ensimmäisen erän kaupallisia akkuja, merkkinä litiumioniakkujen kaupallistamisen alkamisesta. Samaan aikaan, kannettavien elektroniikkalaitteiden markkinat kasvavat nopeasti, vaatii kevyttä ladattavaa akkua. Litiumioniakut, turvallisena ja tehokkaana akuna, on tullut paras valinta.
Kuluneen vuosikymmenen aikana, litiumioniakuista on tullut hallitseva ladattavien akkujen kemiallinen materiaali lähes kaikilla teollisuudenaloilla. Verrattuna aiemmin suosittuihin kemiallisiin materiaaleihin (lyijyakut, nikkeli-kadmium akut, ja alkaliparistot), litiumioniakut ovat monin tavoin parempia. Litium on tällä hetkellä eniten käytetty kemiallinen materiaali, ja muutamilla lisätoiminnoilla, siitä voi tulla turvallisin kemiallinen materiaali. Litiumenergia on aktiivinen tutkimusala, Siksi uusia kemiallisia materiaaleja kehitetään joka vuosi.
Tällä hetkellä, the viisi suurinta maailmanlaajuista litiumioniakkusovellusyritystä are:
Catl (Kiina)
LG Chem (Etelä-Korea)
BYD (Kiina)
Panasonic (Japani)
Samsung Sdi (Etelä-Korea)
2. Litiumioniakun rakenne
2.1 Mikä on a litium-ioni-akku
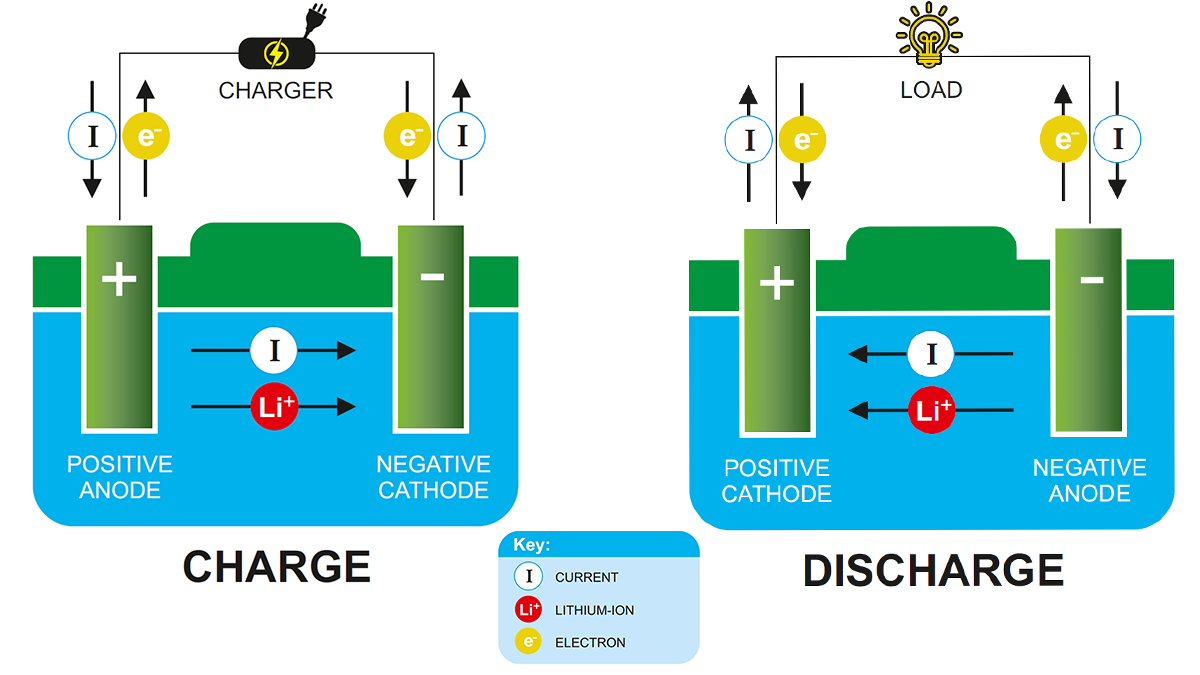
Yksinkertaisesti sanottuna, a litium-ioni-akku viittaa akkuun, jossa on negatiivinen elektrodi (anodi) ja positiivinen elektrodi (katodi), jossa litiumioneja kuljetetaan kahden materiaalin välillä. Litiumioniakkujen toimintaperiaate on sama kuin muiden ladattavien akkujen.
Purkamisen aikana, litiumionit siirtyvät anodilta katodille ja laskeutuvat (upottaa) positiiviseen elektrodiin, joka koostuu litiumista ja muista metalleista. Kun lataus, tämä prosessi on päinvastainen.
Jokaisella litiumioniakulla on jännitealue, joka voi toimia turvallisesti. Alue riippuu akussa käytetyn elektrolyytin kemiallisesta koostumuksesta. Esimerkiksi, LFP-akut ovat 2,5V 0% maksutila (SOC) ja 3,6V at 100% SOC. Tätä pidetään yleensä LFP-akkujen turvallisena toiminta-alueena, kun taas määritellyn alueen alapuolella katsotaan liiallisen purkauksen, ja ylittää määritetyn 100% SOC:ta pidetään ylilatauksena.
2.2 litiumioniakun rakenne
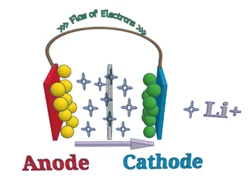
2.2.1 Anodi
Anodi on akun negatiivinen elektrodi. Litiumioniakuissa, anodi koostuu yleensä litiumista ja hiilestä (yleensä grafiittijauhetta). Puhtaus, hiukkaskoko, ja anodimateriaalien tasainen jakautuminen voivat kaikki vaikuttaa niiden kapasiteettiin ja ikääntymisnopeuteen.
2.2.2 Katodi
Katodi on positiivinen elektrodi. Tässä kohtaa erilaiset kemikaalit tulevat peliin. Katodi määrittää litiumin kokonaisenergian kemialliset ominaisuudet. Kuten anodi, keräin on yhdistetty materiaaliin elektronisen reaktiotoiminnan helpottamiseksi. Suurin ero niiden välillä on lämpötila, jossa erilaiset kemikaalit reagoivat elektrolyyttien kanssa (lämpökarkaa) ja niiden tuottaman jännitteen suuruus.
2.2.3 Elektrolyytit
Elektrolyytit mahdollistavat litiumionien siirtymisen ja liikkumisen kahden levyn välillä. Yleensä, se koostuu erilaisista orgaanisista karbonaateista, kuten eteenikarbonaatti ja dietyylikarbonaatti. Eri seokset ja suhteet riippuvat akun käyttöympäristöstä.
Esimerkiksi, matalan lämpötilan sovelluksiin, elektrolyyttiliuoksen viskositeetti on alhaisempi kuin elektrolyyttiliuoksen huoneenlämpötilassa. Litiumparistoissa, litiumheksafluorifosfaatti (LiPF6) on yleisin litiumsuola. Voidaan sanoa, että litiumioniakuissa laajimmin käytetty elektrolyytti on litiumheksafluorifosfaatti (LiPF6), jonka laatu määrää lataus- ja purkamissuorituskyvyn, käyttöelämä, ja litiumioniakkujen turvallisuus.
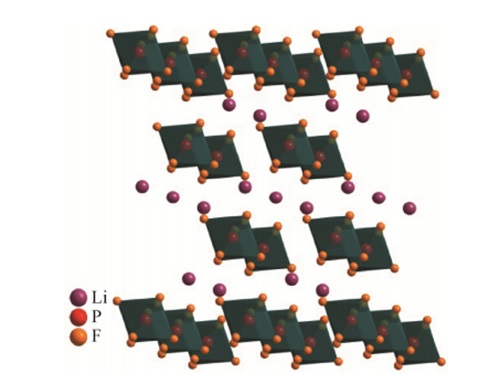
Koska LiPF6:lla on paras kokonaisvaltainen suorituskyky, sillä on erinomainen ympäristöystävällisyys, positiivisen elektrodin virrankeräimen passivointi elektrodin korroosion estämiseksi, ja veteen sekoitettuna, se tuottaa fluorivetyhappoa (HF), mikä edistää SEI-kalvon muodostumista negatiiviselle elektrodille.
SEI on litiummetallin ja elektrolyytin välinen kemiallinen reaktio, joka muodostaa kiinteän elektrolyyttikerroksen litiummetallin pinnalle. Sillä on rooli litiummetallin ja elektrolyytin välisessä eristämisessä ja suojauksessa.
Normaaleissa olosuhteissa, akkuvalmistajat lataavat tyypillisesti hitaasti muodostaakseen yhtenäisen SEI:n hiilianodille.
2.2.4 Kalvo
Litiumioniakkujen erotin on huokoinen muovikalvo, joka helpottaa anodin ja katodin välisen suoran kosketuksen estämistä.. Nämä ohuet kalvot ovat yleensä 20 mikronin paksuiset pienet huokoset päästävät litiumionien läpi lataus- ja purkuprosessien aikana. Kun akku ylittää lämpötila-alueen tai siinä tapahtuu oikosulku, Tämä erotin sulkee huokoset ja estää litiumionien kulkeutumisen läpi, jolloin kemiallinen reaktio pysähtyy.
3. Litiumioniakkujen edut
3.1 Litiumioniakun rakenteen edut
1. Korkean nopeuden purkautuminen, vakaa kapasiteetti
2.Nopea lataus
Litiumioniakut – ladattu sisällä 1 tunnin
Lyijyakut – ohi 9 tuntia
3.Pieni jalanjälki ja vahva kantavuus
4.Useita jaksoja ja pitkä käyttöikä
Litiumioniakut – Jakson käyttöikä on yleensä 5000 ajat, ja täydellinen purkautuminen ei vaikuta syklin käyttöikään
Lyijyakut -300 to 500 ajat, täydellinen purkautuminen vaikuttaa niiden käyttöikään
5.Korkea energiatehokkuus
Litiumioniakut -96% ulostulo, 4% lämpöhäviö
Lyijyakut -15% lämpöhäviö klo 85% ulostulo
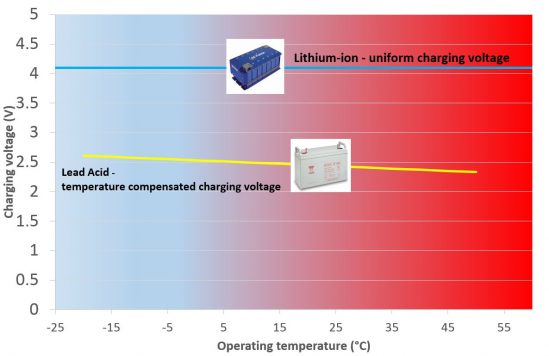
6.Laaja latausjännitevalikoima
Jännitteen kompensointia ei tarvita
7.Vähennä lämmönhallintakustannuksia
Litiumioniakut – hyväksyttävä ilmankierto
Lyijyakku – vaatii ilmastointia
8.Ei kaasupäästöjä
Litiumioniakut – toimivat suljetuissa säiliöissä
Lyijyakut – vaativat vetytuuletuksen
9.Myrkytön, ei kierrätysrajoituksia
Vihreää uutta energiaa, turvallinen ja turvallinen käyttää.
Lisää vertailua litiumakun ja lyijyakun välillä, napsauta nähdäksesi: Litium-akku vs. Lyijyakku yksityiskohtaisempaa ja tarkempaa sisältöä varten.
3.2 Syy lyijyakkujen korvaamiseen litiumioniakuilla
3.2.1 Tehokkuuden parantaminen
Kiitos BMS:n ja lataustekniikan kehityksen, Litiumioniakkuvirtalähdelaitteet voivat auttaa parantamaan tehokkuutta ja vähentämään akkukäyttöisten laitteiden lataamistarpeen aiheuttamia seisokkeja.
3.2.2 Tuottavuuden parantaminen
Operaattoreiden ei tarvitse huolehtia laitteen latausongelmista, ja litiumioniakkuteknologia mahdollistaa yritysten investoinnin automaatioratkaisuihin, vähentää yritysten kustannuksia.
3.2.3 Yksinkertaisempi tapa ladata ja säilyttää
Litiumioniakut voidaan ladata milloin tahansa, mikä tarkoittaa, että voit ladata niitä milloin sinulle sopii. Litiumioniakut eivät myöskään vaadi omaa lataus- tai säilytystilaa, koska ne eivät aiheuta ympäristöriskejä, kuten lyijyakut.
3.2.4 Ei vaadi huoltoa
Toisin kuin lyijyakut, Litiumioniakut eivät vaadi ikäviä tarkastuksia ja huoltomenetelmiä.
3.2.5 Käyttöturvallisuuden parantaminen
Litiumioniakut parantavat tilojen käyttöturvallisuutta eri tavoin, ja ne ovat myös ympäristöystävällisempiä, koska ylikuumenemisriski on pienempi, räjähdys, tai haitallisten kaasujen tai nesteiden päästöjä.
4.1 Tasapainoinen lataus
Ylilataa akku normaalin jännitteen yläpuolella täydellisen latausjakson jälkeen. Tämä vaihe on välttämätön, jotta voidaan poistaa kertyneet sulfaatit ja tasapainottaa kunkin akun jännite lyijyakkuissa.
4.2 Akun heikkeneminen
Prosessi, jolla vähennetään akun varastoiman energian määrää. Lämpötila, lataus- ja purkujännite, ja lataus- ja purkaussyvyys voivat vaikuttaa siihen, kuinka paljon akun kapasiteetti pienenee ajan myötä.
4.3 Akun käyttöjaksojen määrä
Jos akku on latautunut ja purkautunut syklinä, latausten ja purkausten kumulatiivinen määrä. Akun sykli koostuu 100% purkaminen ja lataus.
4.4 Akun kesto
Kuinka kauan akkua voidaan käyttää sen käyttöiän aikana. Elinikä mitataan täydellisten lataus- ja purkujaksojen lukumäärällä.
4.5 Työlämpötila
Hyväksyttävä lämpötila ympäristössä, jossa akku toimii. Jos työlämpötila ylittää alueen, akku saattaa epäonnistua.
4.6 UL-listaus/sertifiointi
UL-luettelo/sertifiointi tarkoittaa, että UL on arvioinut tuotenäytteet varmistaakseen, että ne täyttävät erityisvaatimukset. Tämä sisältää testausnäytteet, jotka kattavat litiumioniakkurakenteen toiminnallisen turvallisuuden ja käyttötapaukset.
5. Miksi valita litiumionit?- Analyysi litiumioniakun rakenteen näkökulmasta

5.1 Erinomainen laatu
Litiumioniakkujen suuri energiatiheys ja laajat purkausjaksot ovat tärkeimpiä tekijöitä, joten ne ovat välttämättömiä monissa laitteissa. Ja ne ovat myös monilta muilta osin parempia kuin perinteinen akkukemia. Akkujen korkean energiatiheyden lisäksi, ne voivat myös purkaa suurella teholla ja ladata nopeasti. Tämä antaa niille suuremman toiminnan joustavuuden kuin lyijyakut.
Sovellusskenaarioissa, joissa latausteho tai latausaika on tiukka, kuten aurinkosähköjärjestelmät, jatkuva käyttö osittain ladatussa tilassa ei vahingoita litiumioniakkuja.
5.2 Ympäristöystävällinen
Litiumioniakkujen ja ympäristön välinen vuorovaikutus on erittäin lievää. Latauksen aikana ei synny haitallisia kaasuja, ja lämpöhäviö on erittäin pieni. Tämä tarkoittaa, että litiumioniakkuja voidaan käyttää suljetuissa tiloissa, täysin eristetty ympäristöstä. Myös käytettyjen paristojen kierrätys ja uudelleenkäyttö on erittäin kätevää, koska ne eivät sisällä myrkyllisiä aineita, kuten kadmiumia, elohopeaa, ja johtaa.
5.3 Useita rakenteita
Purkamisen aikana, varaus liikkuu ulkoisen piirin läpi akkuelektrodien välillä. Tasapainottaakseen latauksen siirtoa akun sisällä, Positiivisesti varautuneet litiumionit liikkuvat sisäisen elektrolyyttipiirin läpi positiivisen ja negatiivisen elektrodin välillä. Kun lataus, Prosessi kääntyy, ja litiumionit palaavat elektrolyytin läpi.
Katodeina voidaan käyttää monenlaisia kemikaaleja (katodit) valmistaa litiumioneja kuljettavia elektrodimateriaaleja. Elektrolyyttimateriaalit ovat myös tutkimussuunta, ja materiaalien, kuten kiinteiden aineiden ja nesteiden tila on myös tutkimusaihe. Tämä on erittäin aktiivinen tutkimus- ja kehitysala, mikä edistää litiumioniakkujen kehitystä yhä useammissa markkinasovelluksissa.
Johtopäätös
Nyt kun olet ymmärtänyt niin paljon analyysiä ja dataa, sinulla pitäisi olla tietty käsitys litiumakuista: heidän historiaansa, etuja, ja kehityssuunta.
Sähkötuotteiden tulevaisuus on saapunut. Perinteisestä energiasta uuteen energiaan siirtymisen kysyntää ei voida sivuuttaa. Yhä useammat teollisuudenalat ja yritykset ymmärtävät litiumioniakkuteknologian edut, Päätösten tekeminen liiketoiminnan painopisteen siirtämiseksi on tullut helpommaksi.
Ota meihin yhteyttä, GYCX yhden luukun palvelu tarjoaa täydellisen ratkaisun tarpeisiisi.
Aiotteko investoida litiumioniakkuteknologiaan nyt??
FAQ
1. Voivatko litiumparistot korvata alkaliparistot?
Vaikka litiumakut käyttävät kalliimpaa akkutekniikkaa, Niiden kyky ylläpitää korkeaa jännitettä tarkoittaa, että ne ovat erinomainen vaihtoehto alkaliparistoihin.
2.Vuotaako litiumakut?
Litiumparistot eivät vuoda, joten ne ovat erittäin turvallisia säilyttää.
Litium voi syttyä tuleen joutuessaan kosketuksiin ilman tai veden kanssa. Ne vuotavat vähemmän todennäköisesti nestemäisten elektrolyyttien vuoksi, pakokaasujen käsittelytekniikka on jo hyvin kypsä.
3.Missä lämpötilassa litiumioniakut räjähtävät?
Litiumakut voivat räjähtää 538 celsiusastetta.
Jos litiumakkua kuumennetaan pitkään, se voi. Koska litiumioniakuilla on erittäin korkea energia, kun ne kuumenevat, ne vapauttavat orgaanisia liuottimia, jotka vaikuttavat elektrolyyttiin; Tämä lämpö voi saada ne räjähtämään.
Oikosulut, jotka syntyvät akun napojen joutuessa kosketuksiin metallin kanssa, voivat myös aiheuttaa räjähdyksiä.
